FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium
Bracco Diagnostics Inc. has announced that the U.S. Food and Drug Administration (FDA) granted import discretion of Iomeron (iomeprol injection) into the U.S. to address the ongoing iodinated contrast media shortage. The product addresses the need for the most advanced diagnostic imaging standards and will be temporarily available in the U.S. market starting at the end of August, 2022.
CT Angiography (CTA) Imaging Technology News

FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium Iomeron (iomeprol injection) to Address Supply Shortages
articles • APPLIED RADIOLOGY
Fresenius Kabi Further Expands Radiology Portfolio with Launch of Gadobutrol Injection
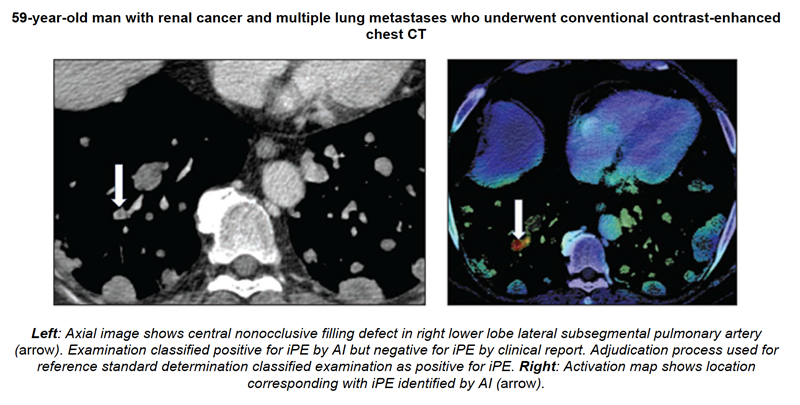
articles • APPLIED RADIOLOGY

Bracco Announces FDA Approval of Gadopiclenol Injection, a New Macrocyclic High-Relaxivity Gadolinium-Based Contrast Agent which will be commercialized as VUEWAY™ (gadopiclenol) injection and VUEWAY™ (gadopiclenol) Pharmacy Bulk Package by Bracco

Survey Reports Radiologist Input on Key Issues, MRI Contrast Agents

Guerbet Announces ACR Committee on Drugs and Contrast Media Classifies Elucirem (Gadopiclenol) Injection a Group II Agent
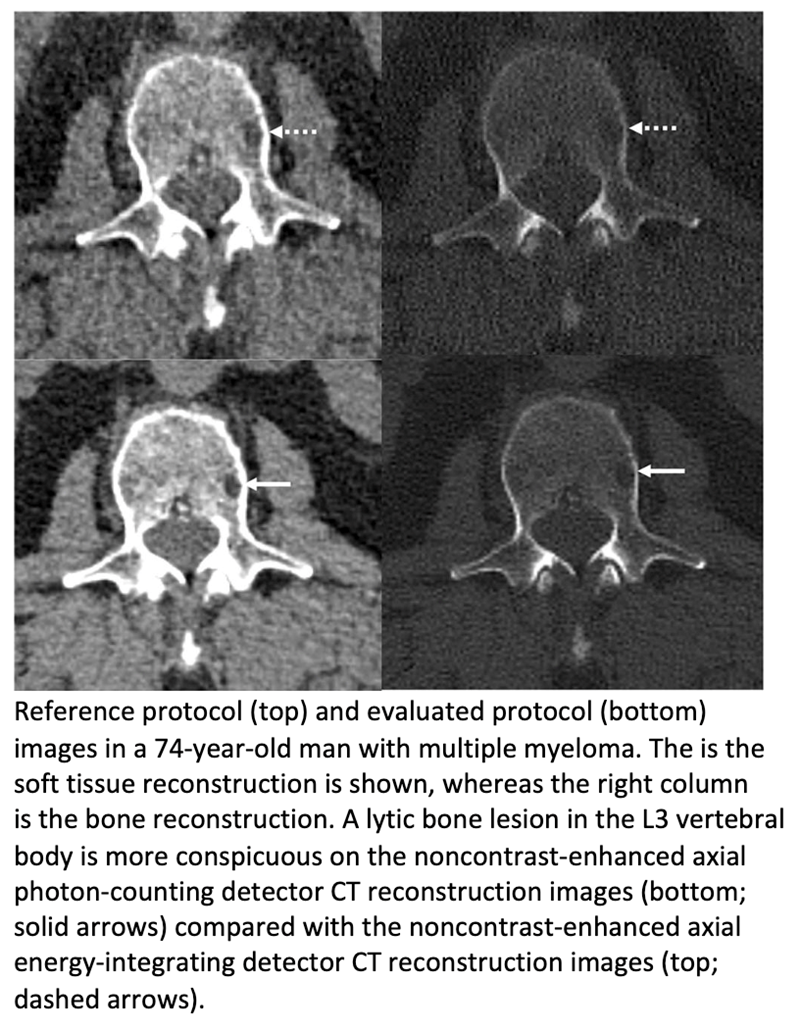
articles • APPLIED RADIOLOGY