
How can graphite and diamond be so different if they are both composed of pure carbon?
4.6
(630)
Write Review
More
$ 30.50
In stock
Description
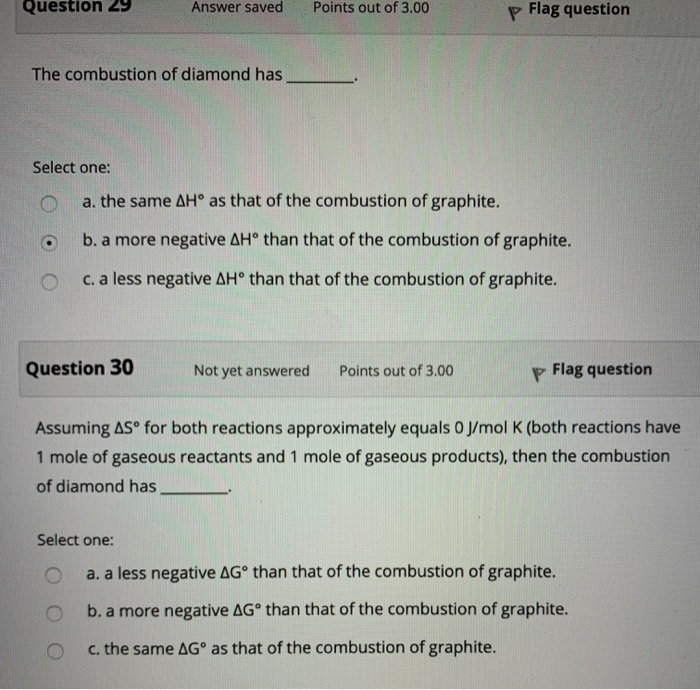
Solved Diamond and graphite are both made from pure carbon;

Graphite: Properties, Occurrence and Applications - Matmatch
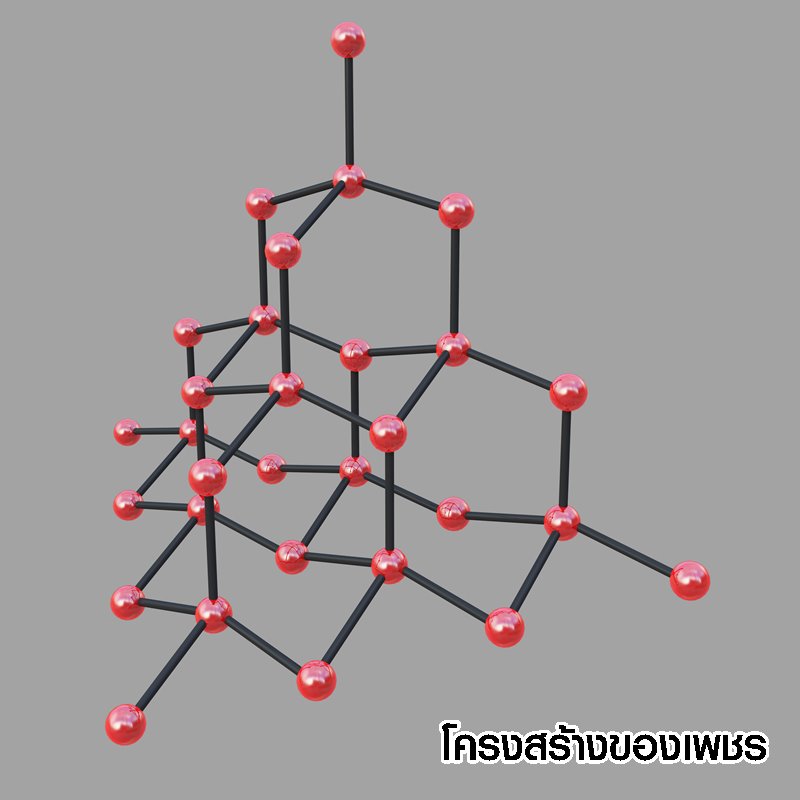
คาร์บอน (C) ธาตุพื้นฐานของชีวิต

The Atomic Difference Between Diamonds and Graphite – Sustainable Nano
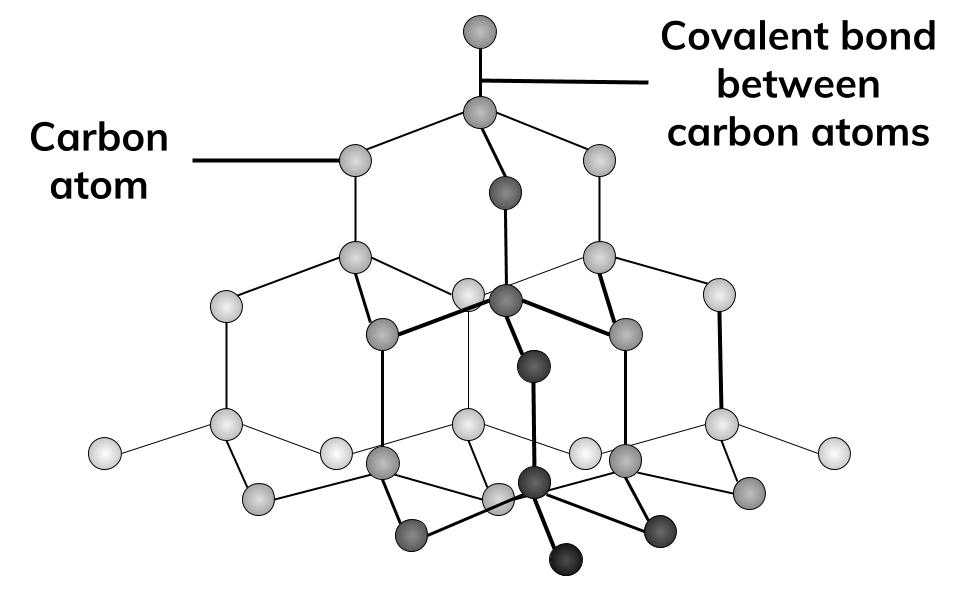
1:50 explain how the structures of diamond, graphite and C60 fullerene influence their physical properties, including electrical conductivity and hardness - TutorMyself Chemistry

Realmente se pueden hacer DIAMANTES comprimiendo CARBÓN?

Structure of Diamond and Graphite - Differences and Similarities

How is graphite different from diamonds? - Noon Academy

What happens to Air bearing systems when the air supply suddenly

Blog OAV Air Bearings

How pencils can help us understand the future of everything (Alex
Related products
You may also like









